Abstract
BACKGROUND: Richter's syndrome (RS), an aggressive lymphoma in a chronic lymphocytic leukemia (CLL) patient, has a poor prognosis and new therapies are needed. Recently, a clinical trial of the programmed death receptor-1 (PD-1) blocking antibody, pembrolizumab, in relapsed CLL and RS reported responses in RS. Two PD-1 inhibitors, nivolumab and pembrolizumab, are approved by the US FDA for other cancers. Based on reported responses in RS with pembrolizumab and the favorable toxicity profile of these agents we have prescribed them for RS outside of clinical trials. We reviewed our institutional experience.
METHODS: Medical records of all patients with a diagnosis of RS treated outside of a clinical trial with at least 1 dose of either pembrolizumab or nivolumab at the Ohio State University (OSU) were reviewed. Baseline characteristics, CLL and RS disease features, and treatment were abstracted from medical records. Treatment failure was defined as disease progression documented by the treating physician, start of a new treatment for RS other than planned allogeneic stem cell transplant, or death while taking a PD-1 inhibitor. Time to treatment failure was calculated from initiation of a PD-1 inhibitor. Patient and disease characteristics were described using summary statistics and time to treatment failure and overall survival were estimated using the method of Kaplan-Meier.
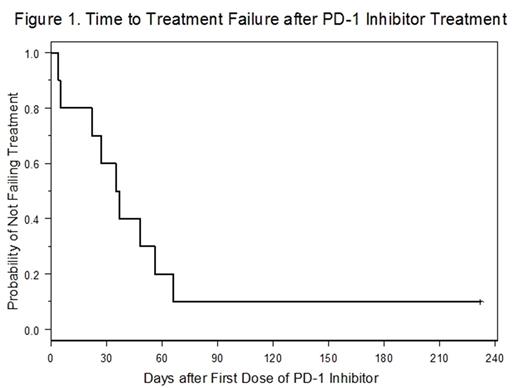
RESULTS: A total of 10 patients received PD-1 inhibitors for RS. The median age was 69 (range 52-79) and 4/10 (40%) were men. The median time from CLL diagnosis to biopsy demonstrating RS was 8.1 (range 1-14) years. The cohort was enriched for high-risk genetic features in the underlying CLL: 8/10 (80%) IGHV unmutated, 7/10 (70%) deletion 17p by FISH, and 5/10 (50%) complex CLL karyotype. Patients had a median of 3.5 (range 1-11) treatments for CLL prior to RS diagnosis and all 10 had prior BTK inhibitor exposure. All patients had biopsy proven RS with diffuse large B-cell lymphoma histology. The PD-1 inhibitor used was nivolumab in 7/10 (70%) patients and pembrolizumab in 3/10 (30%). The majority of patients (6/10, 60%) received a PD-1 inhibitor as an initial treatment for RS, and the median time from RS biopsy to first dose of PD-1 inhibitor was 27 (range 13-86) days. Three patients had 1 prior RS treatment: R-EPOCH, rituximab and high-dose methylprednisolone, or radiation therapy, respectively. One patient was more extensively pre-treated and had received hyper-CVAD, pulse dexamethasone, radiation therapy, and single-agent rituximab. For patients that received nivolumab the total number of doses was known for 6/7 (86%) and the median doses received was 2.5 (range 1-5). For patients treated with pembrolizumab the number of doses received was known for 2/3 (67%) patients with 1 patient receiving only 1 dose and 1 receiving 10 doses. One patient in each drug group received doses outside of OSU. In 4/10 (40%) cases a targeted therapy for CLL was given concurrently with the PD-1 inhibitor. This was single agent ibrutinib in 3 cases and combination ibrutinib and venetoclax in 1. Two of these patients were taking the targeted therapy at the time of developing RS and the PD-1 inhibitor was added. Therapy outcome was known for all 10 patients. The majority of patients (9/10, 90%) had treatment failure: 3 had disease progression, 2 started a new therapy due to lack of response, and 4 died prior to documentation of response. Median time to treatment failure from the first dose of PD-1 inhibitor was 36 days (95% CI: 4-56 days) (Figure 1). Only 1 patient received a sustained course of a PD-1 inhibitor (pembrolizumab) and underwent allogeneic stem cell transplant without experiencing RS relapse. That patient had disease features distinct from the rest of the cohort in that the only site of RS was brain parenchyma and they had a complete resection at time of diagnosis. Median overall-survival for the cohort from RS biopsy was 123 (95% CI: 33-not reached) days with median follow-up of 125 days and 4/10 (40%) of patients were alive at last follow-up.
CONCLUSIONS: Review of our institutional experience with the use of PD-1 inhibitors for the treatment of RS demonstrates poor efficacy with a very short time to treatment failure. This may be due to the aggressive nature of RS or a highly enriched population of patients already on immune modulatory therapy with ibrutinib. Our data do not support the use of monotherapy PD-1 inhibitors outside of clinical trials for RS.
Byrd: Genentech: Research Funding; Janssen: Research Funding; Acerta Pharma: Research Funding; Pharmacyclics: Research Funding; The Ohio State University: Patents & Royalties: OSU-2S.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal